The repressilator design objectives¶
From the analysis we can extract several design objectives or guidelines that will optimize the chances of achieving self-sustaining oscillations. An ideal system should use:
Low "leakiness:" $\rho \ll 1$ ⟶ Use tight, artificial promoters that can be fully repressed.
Strong promoters: $\beta \gg 1$ ⟶ Can be achieved using strong promoters derived from phages that produce high protein levels
Similar protein and mRNA decay rates $\gamma\approx 1$ ⟶ Destabilize repressors to increase their decay rates to be more comparable to those of mRNA. This can be done by adding destabilizing C-terminal tags based on the ssrA protein degradation system.
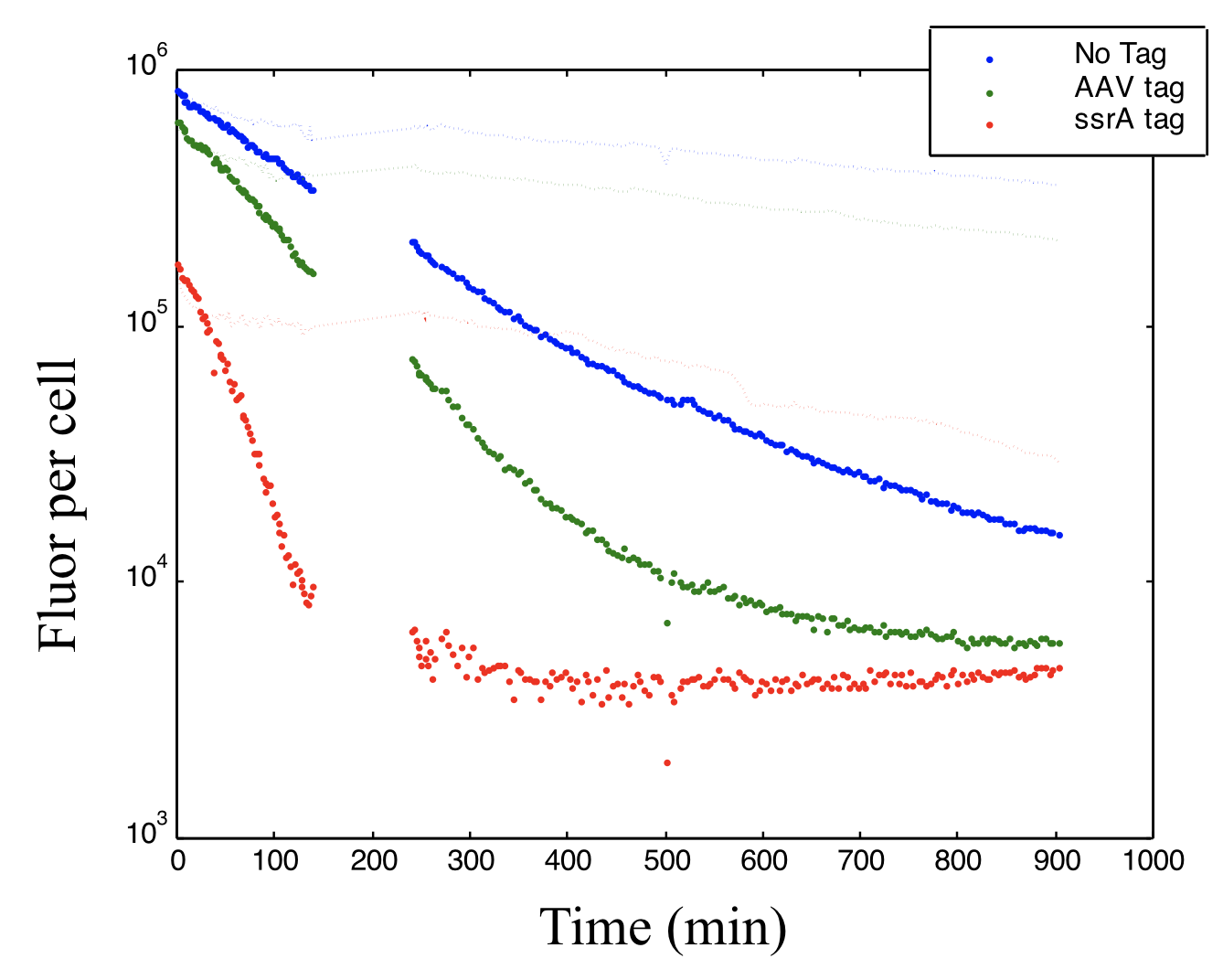
The protein production shut off was at time zero. This plot shows how adding different variants of the 11-amino acid ssrA tag can alter the decay rate of a fluorescent protein. The gap in data due to technical glitch.
- Ultrasensitive repression curves, ideally $n > 1.5$ or $2$, or as large as possible ⟶ Use intrinsically cooperative repression mechanisms, such as those from phage λ, or those that incorporate multiple binding sites, such as those in the TetR system. Lutz & Bujard showed that the phage λ $P_R$ promoter architecture provides a high regulatory range, and can be adapted to work with binding sites for LacI and TetR.
Additional biological design goals:
To minimize toxicity from overexpressing repressors, put the circuit on a low copy plasmid (pSC101)...
...But to maximize the readout, put a fluorescent reporter gene on a higher copy number plasmid (ColE1).
Destabilize the fluorescent protein so that it can track the circuit activity
Avoid "read through" from one operon to the next ⟶ add transcriptional terminators between promoter-repressor units.
Based on these considerations, the repressilator was designed as a two plasmid system to be used in an E. coli strain deleted for the natural lac operon.
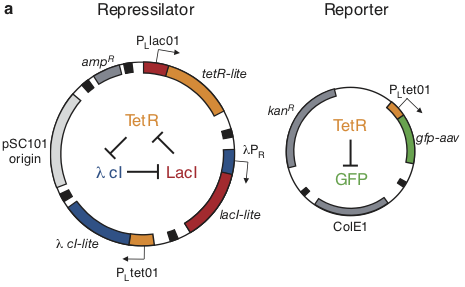
Here, the repressilator consists of three repressors on the low copy pSC101 plasmid, with TetR additionally repressing a green fluorescent protein reporter on the higher copy ColE1 plasmid. The lite suffix on the repressors signifies that they have a destruction tag to decrease their stability. The aav suffix on the GFP indicates that it is a variant of intermediate stability.