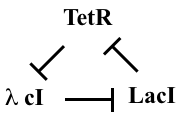
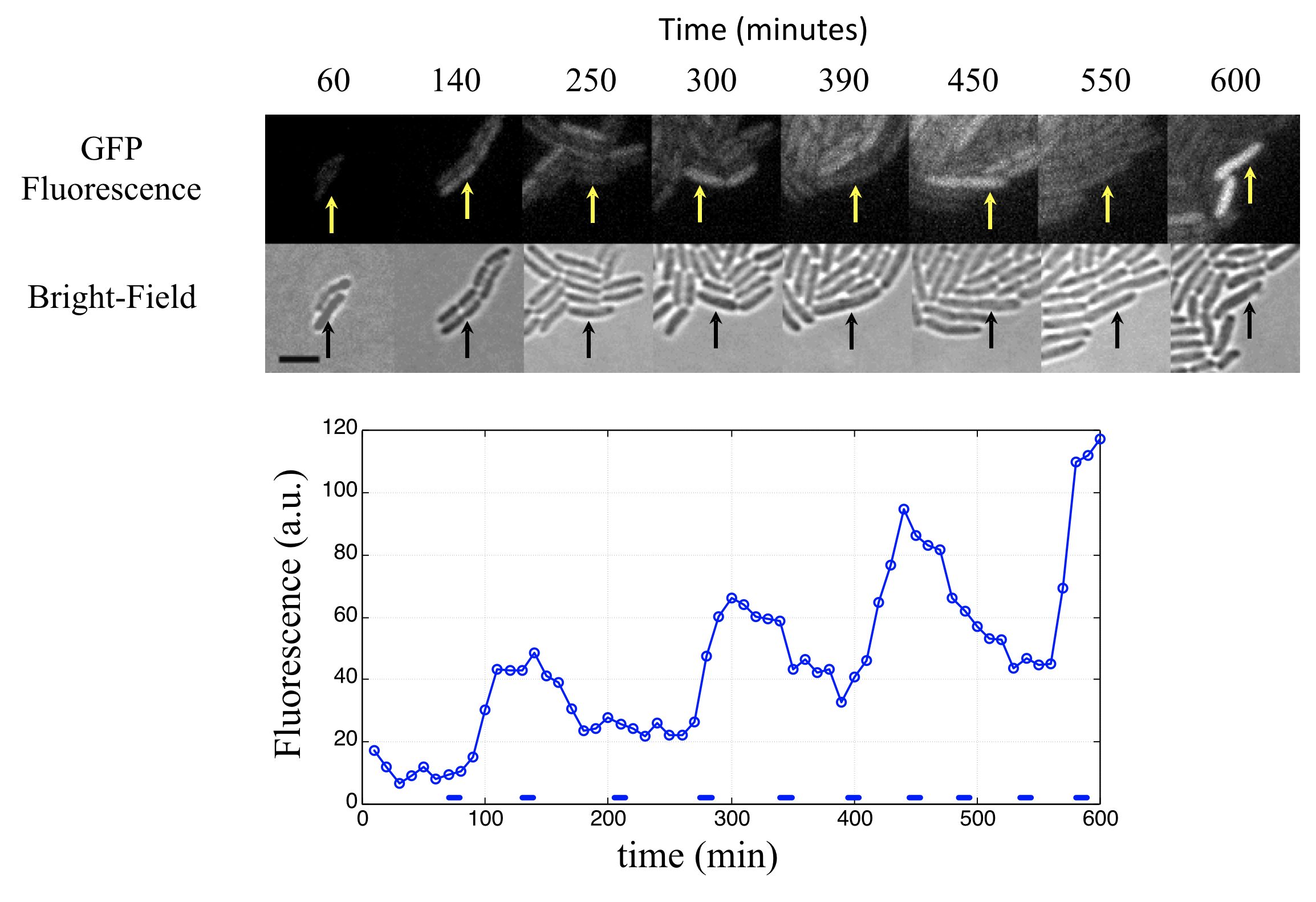
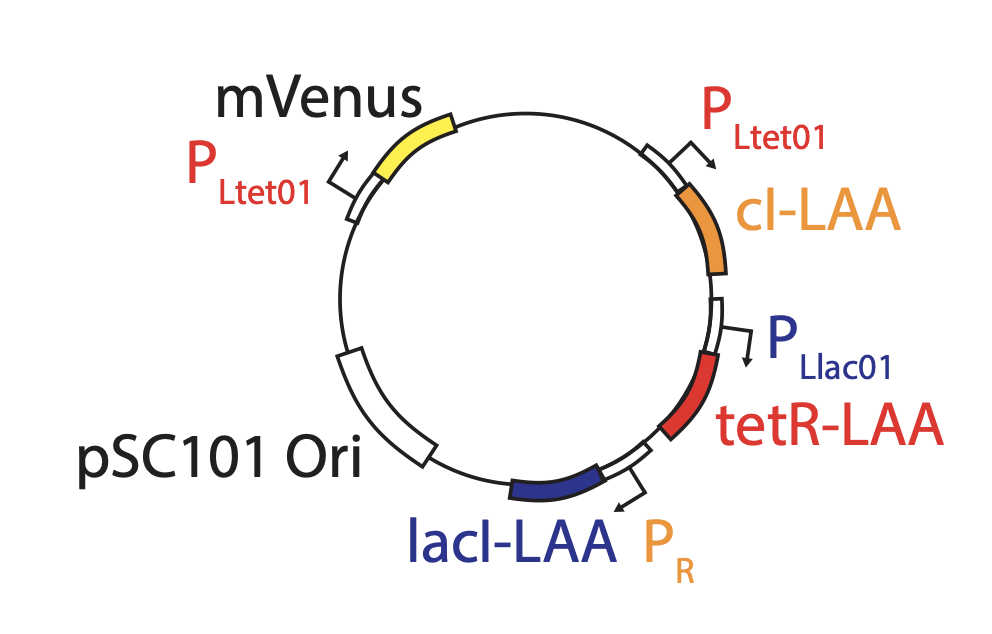
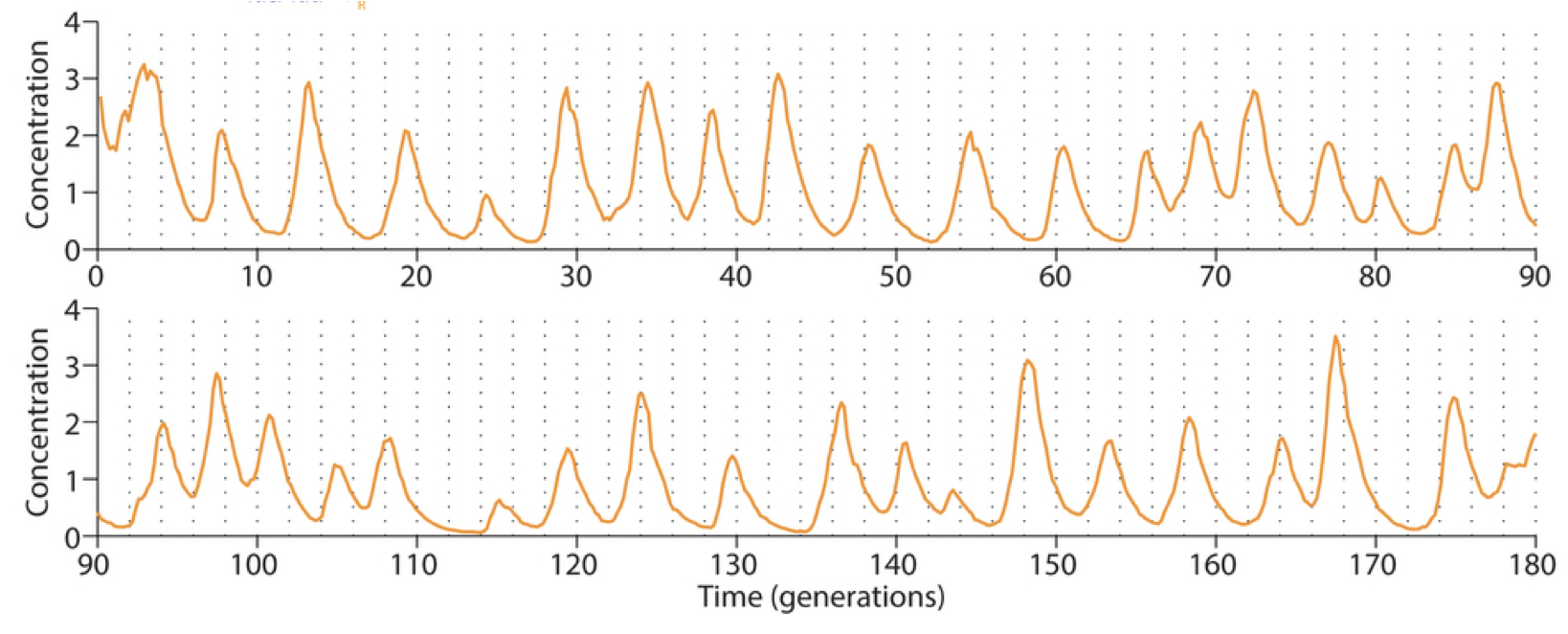
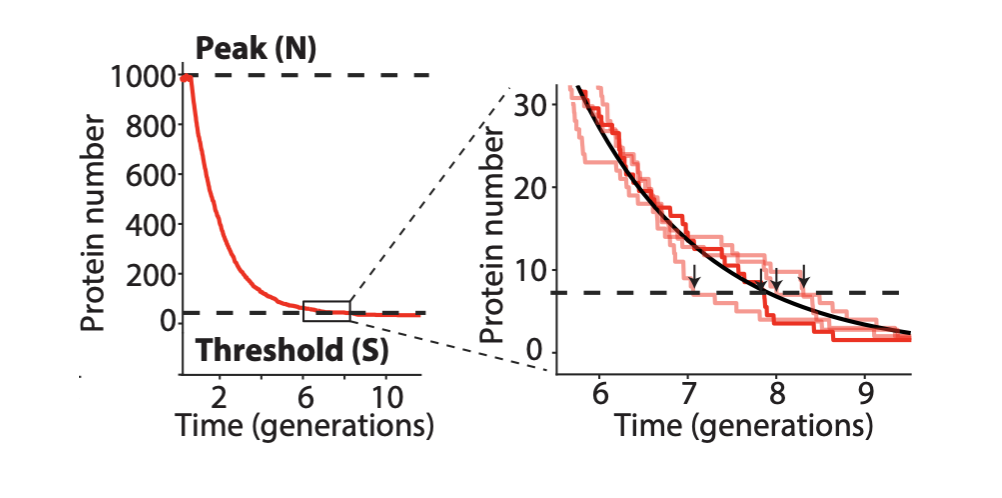
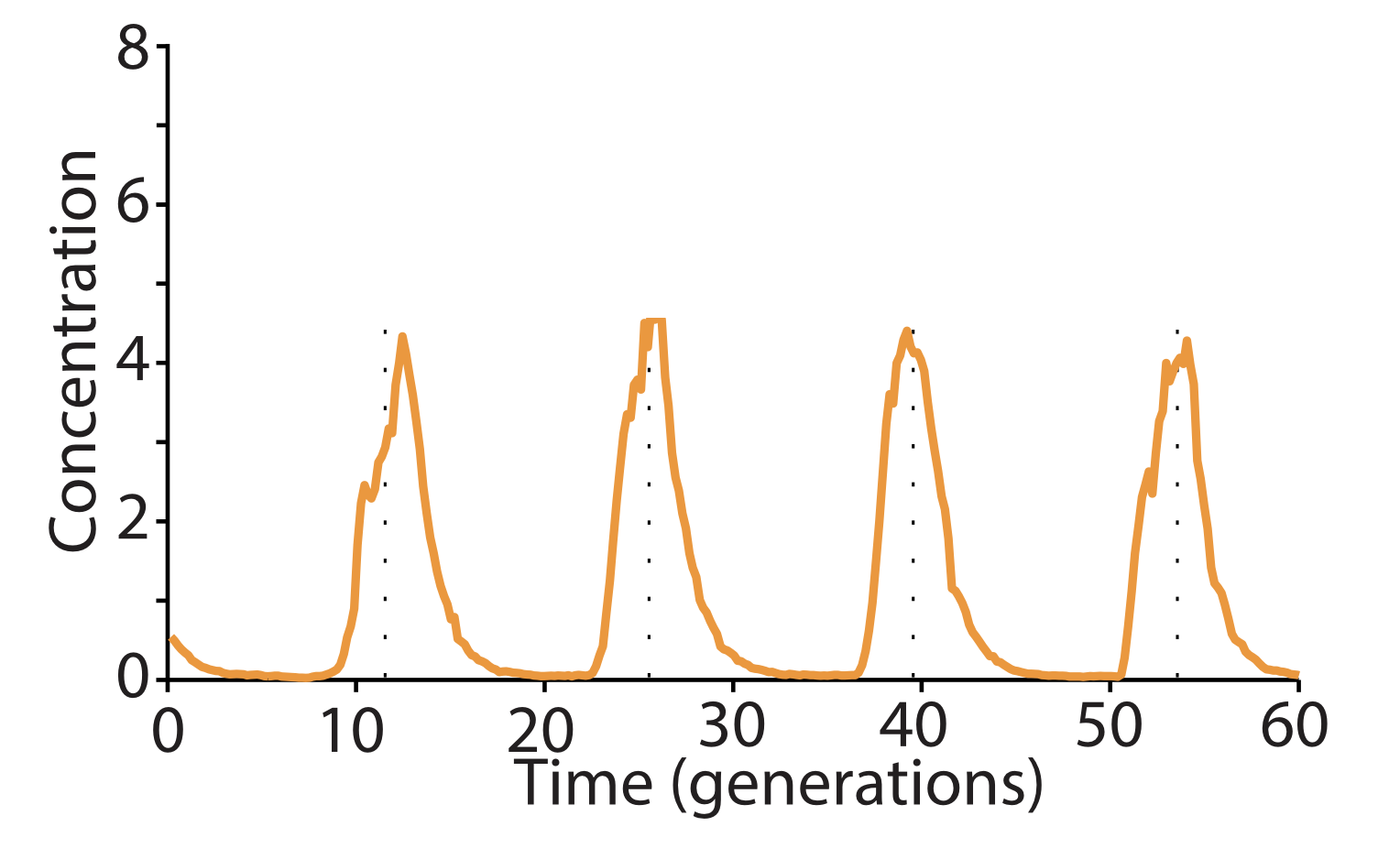
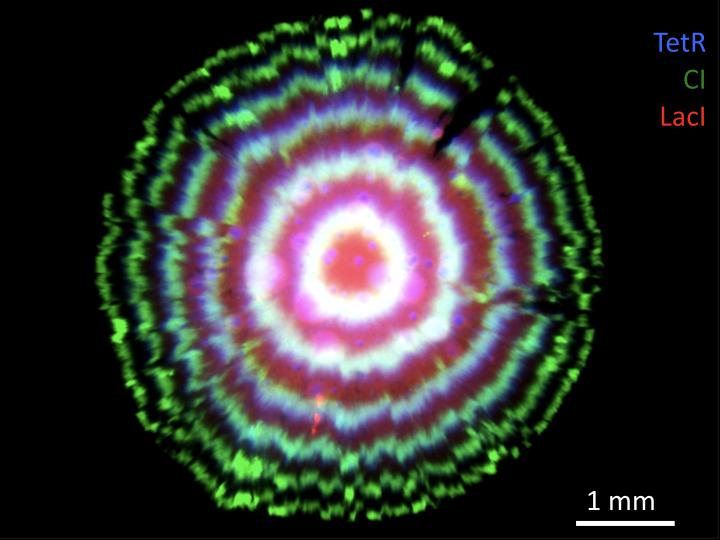
Linear stability analysis¶
We first give a minimal introduction to the technique of linear stability analysis. The basic idea is that to approximate a nonlinear dynamical system by its Taylor series to first order near the fixed point and then look at the behavior of the simpler linear system. The Hartman-Grobman theorem (which we will not derive here) ensures that the linearized system faithfully represents the phase portrait of the full nonlinear system near the fixed point.
Say we have a dynamical system with variables $\mathbf{u}$ with
\begin{align}
\frac{\mathrm{d}\mathbf{u}}{\mathrm{d}t} = \mathbf{f}(\mathbf{u}),
\end{align}
where $\mathbf{f}(\mathbf{u})$ is a vector-valued function, i.e.,
\begin{align}
\mathbf{f}(\mathbf{u}) = (f_1(u_1, u_2, \ldots), f_2(u_1, u_2, \ldots), \ldots).
\end{align}
Say that we have a fixed point $\mathbf{u}_0$. Then, linear stability analysis proceeds with the following steps.
1. Linearize about $\mathbf{u}_0$, defining $\delta\mathbf{u} = \mathbf{u} - \mathbf{u}_0$. To do this, expand $f(\mathbf{u})$ in a Taylor series about $\mathbf{u}_0$ to first order.
\begin{align}
\mathbf{f}(\mathbf{u}) = \mathbf{f}(\mathbf{u}_0) + \nabla \mathbf{f}(\mathbf{u}_0)\cdot \delta\mathbf{u} + \cdots,
\end{align}
where $\nabla \mathbf{f}(\mathbf{u}_0) \equiv \mathsf{A}$ is the Jacobi matrix,
\begin{align}
\nabla \mathbf{f}(\mathbf{u}_0) \equiv \mathsf{A} = \left.\begin{pmatrix}
\frac{\partial f_1}{\partial u_1} & \frac{\partial f_1}{\partial u_2} & \cdots \\[0.5em]
\frac{\partial f_2}{\partial u_1} & \frac{\partial f_2}{\partial u_2} & \cdots \\
\vdots & \vdots & \ddots
\end{pmatrix}\right|_{\mathbf{u}_0}.
\end{align}
Thus, we have
\begin{align}
\frac{\mathrm{d}\mathbf{u}}{\mathrm{d}t} = \frac{\mathrm{d}\mathbf{u}_0}{\mathrm{d}t} + \frac{\mathrm{d}\delta\mathbf{u}}{\mathrm{d}t}
= \mathbf{f}(\mathbf{u}_0) + \mathsf{A} \cdot \delta\mathbf{u} + \text{higher order terms}.
\end{align}
Since
\begin{align}
\frac{\mathrm{d}\mathbf{u}_0}{\mathrm{d}t} = \mathbf{f}(\mathbf{u}_0) = 0,
\end{align}
we have, to linear order,
\begin{align}
\frac{\mathrm{d}\delta\mathbf{u}}{\mathrm{d}t} \approx \mathsf{A} \cdot \delta\mathbf{u}.
\end{align}
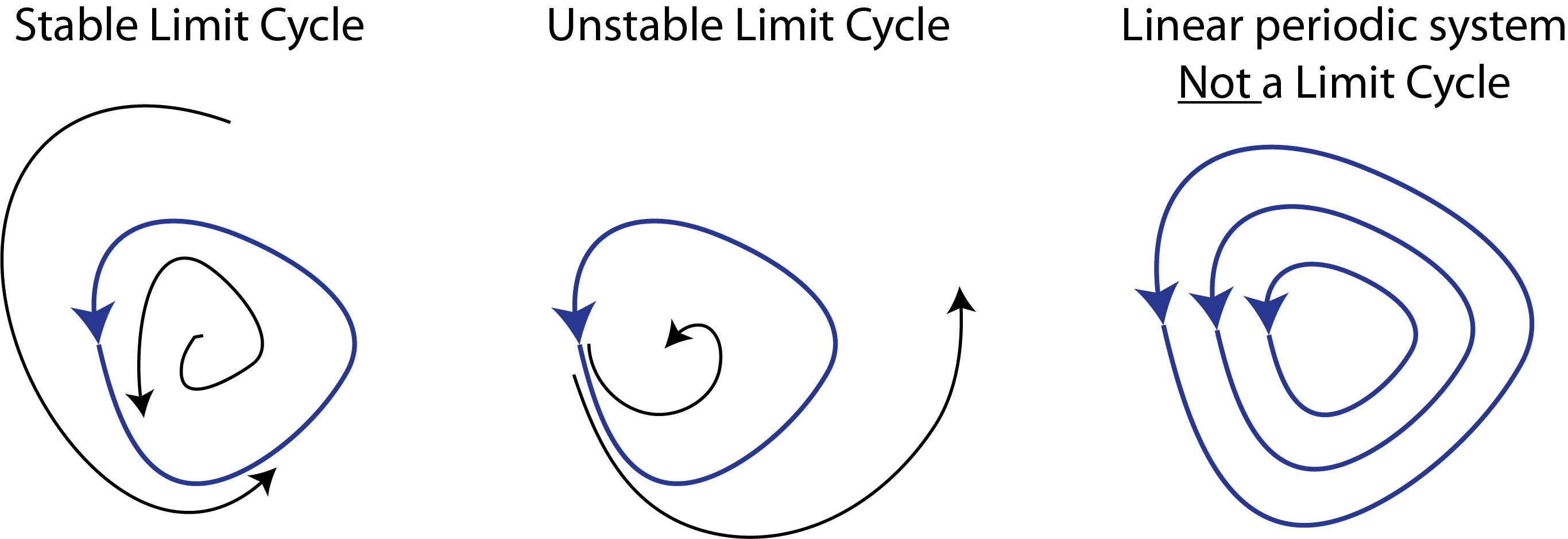
This is now a system of linear ordinary differential equations. If this were a one-dimensional system, the solution would be an exponential $\delta u \propto \mathrm{e}^{\lambda u}$. In the multidimensional case, the growth rate $\lambda$ is replaced by a set of eigenvalues of $\mathsf{A}$ that represent the growth rates in different directions, given by the eigenvectors of $\mathsf{A}$
2. Compute the eigenvalues $\lambda$ of $\mathsf{A}$.
3. Determine the stability of the fixed point using the following rules.
- If $\mathrm{Re}(\lambda) < 0$ for all $\lambda$, then the fixed point $\mathbf{u}_0$ is linearly stable.
- If $\mathrm{Re}(\lambda) > 0$ for any $\lambda$, then the fixed point $\mathbf{u}_0$ is linearly unstable.
- If $\mathrm{Im}(\lambda) \ne 0$ for a linearly unstable fixed point, the trajectories spiral out, potentially leading to oscillatory dynamics.
- If $\mathrm{Re}(\lambda) = 0$ for one or more $\lambda$, with the rest having $\mathrm{Re}(\lambda) < 0$, then the fixed point $\mathbf{u}_0$ lies at a bifurcation.
So, if we can assess the dynamics of the linearized system near the fixed point, we can get an idea what is happening with the full system.
To do the linearization, we need to do Taylor expansions of Hill functions. We do this so often in this course, that we will write them here and/or memorize for future use.
\begin{align}
\frac{x^n}{1+x^n} &= \frac{x_0^n}{1+x_0^n} + \frac{n x_0^{n-1}}{(1+x_0^n)^2}\,\delta x + \text{higher order terms}, \\[1em]
\frac{1}{1+x^n} &= \frac{1}{1+x_0^n} - \frac{n x_0^{n-1}}{(1+x_0^n)^2}\,\delta x + \text{higher order terms}.
\end{align}
In the following we only need the second, repressing case.